Inspiring New Options
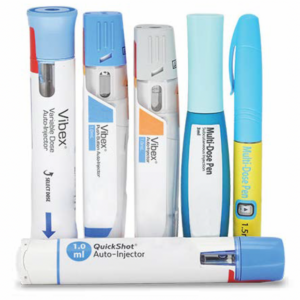
Partner Auto-Injector Pipeline
Our auto-injector technology is globally validated and is used in five approved therapeutics, providing improved dose administration and treatment options, with additional products currently in development.
The content below contains information on investigational products and uses that have not been approved by the U.S. Food and Drug Administration. This information is presented only for purposes of providing a general overview of development programs and should be construed as conclusions of efficacy, safety, or future approval of any product.
[auto-iframe link=https://halozyme.com/partner-auto-injector-pipeline-build/]
Are you interested in learning more about our partnerships and business development opportunities? Contact bd@halozyme.com.
Halozyme Auto-Injector Pipeline
[auto-iframe link=https://halozyme.com/halozyme-auto-injector-pipeline-build/]